dialysis involves movement of molecules across a membrane. Clinically, this movement takes place in and out of blood, across a membrane. If the blood is exposed to an artificial membrane outside of the body, the process is semipermeable called hemodialysis or hemofiltration. If the exchange of molecules occurs across the peritoneal membrane, the process is called PD. semiperme
Diffusion : two solutions of different concentrations are separated by a semipermeable membrane, solute will move from the side of higher to the side of lower solute concentration. This process of solute movement on a concentration gradient is called diffusion and is caused by the random movement of the solute molecules, striking and moving across the membrane. Several factors influence this random movement and thus the rate of diffusion. The transport of any solute or solvent molecule is dependent on the physical size of the molecule relative to the size of the pores in the membrane. Any molecules larger than the pores of the membrane cannot pass through. Similarly, the electrical charge and the shape of the molecule also determine the rate of transport across the membrane. If the membrane has a negative charge, particles with a like charge will have limited transport as compared with those with a positive or a neutral charge. ( in other words shorts term higher diffusion concentration to lower concentrations due to random molecules & motion
Mechanisms Involved in Molecule Movement: The movement of molecules follows certain physiological and physicochemical principles that are outlined below
Osmosis : As solute concentration increases, solvent concentration correspondingly decreases and vice versa. If a semipermeable membrane separates solutions of different concentrations, solvent will flow from the side with the higher solvent concentration to the side with the lower solvent concentration. This process is called osmosis
Ultrafiltration : A solvent such as water can be forced across a semipermeable membrane on a pressure gradi- ent. Thus, solvent molecules will move from higher to lower pressures The pressure could be a result of osmotic force (see above) or of mechanical hydrostatic pressure. This movement of solvent (water) molecules across a semipermeable membrane, caused by a—–
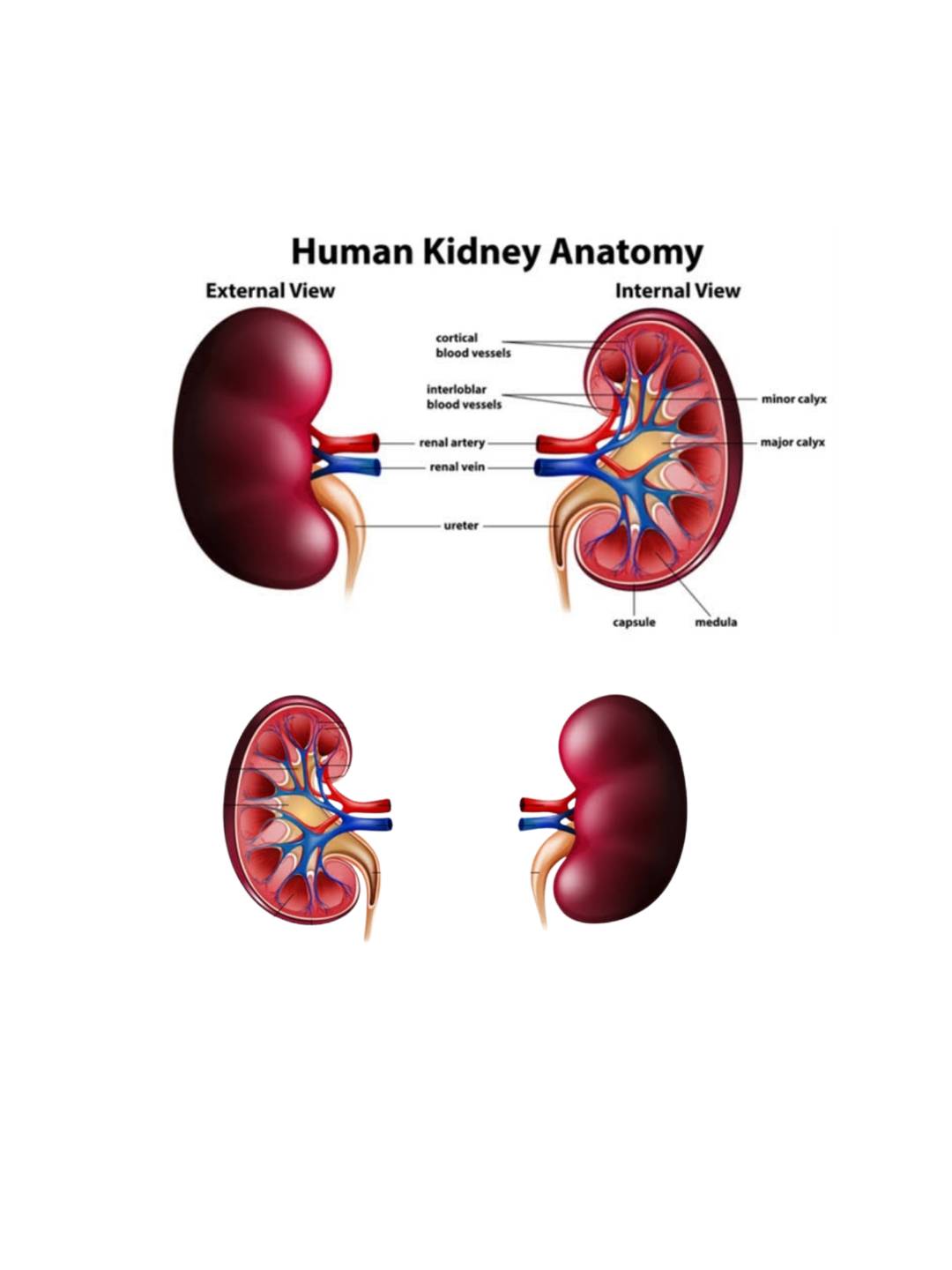
 .
.
by R Ali – Rahmani
any help/ idea contact on email: syedrizwanali602@gmail.com
thank you for visiting


